TREOCAPA: Prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen
Overview
The TREOCAPA clinical trial deals with the effective prophylaxis (a measure to prevent a disease) of a persistent ductus arteriosus (PDA) (see next paragraph below), which often occurs in very preterm born children. The trial is conducted in approximately 65 Neonatal Intensive Care Units (NICUs) in 17 European countries. Preterm infants of a gestational age of 23 to 28 weeks are included in the study. INSERM, a biomedical French research institute, is the sponsor of the trial which is led by Nantes University Hospital in France. The TREOCAPA trial is a Proof of Viability study of the Conect4Children (c4c) project, which aims to develop a Pan European Paediatric Clinical Trial Network.
What is a persistent ductus arteriosus?
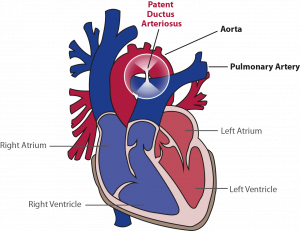 The ductus arteriosus is the vessel, that communicates the aorta (the main and largest artery in the body, that distributes blood enriched with oxygen to all parts of the body) and pulmonary artery (the artery carrying blood from the heart to the lungs where the blood is enriched with oxygen) when the baby is still in the womb. It is present in all babies before birth and normally closes in the days following birth. However, in about 60% of preterm born babies, this vessel remains open. This is most often the case in the more premature ones. If this vessel does not close, babies often experience more complications of prematurity with the lungs, brain, and gut than babies, in which this vessel does close.
The ductus arteriosus is the vessel, that communicates the aorta (the main and largest artery in the body, that distributes blood enriched with oxygen to all parts of the body) and pulmonary artery (the artery carrying blood from the heart to the lungs where the blood is enriched with oxygen) when the baby is still in the womb. It is present in all babies before birth and normally closes in the days following birth. However, in about 60% of preterm born babies, this vessel remains open. This is most often the case in the more premature ones. If this vessel does not close, babies often experience more complications of prematurity with the lungs, brain, and gut than babies, in which this vessel does close.
Why TREOCAPA?
There are certain drugs (indomethacin or ibuprofen) that can be used to close the ductus arteriosus. As these drugs have many adverse effects, their prophylactic use (that means using the drug in advance, before anyone knows if the ductus will close without intervention or not) is not recommended. Recently, it has been shown that paracetamol, a drug with far fewer side effects, can also close the ductus arteriosus. This drug is widely used in neonatology (the care of newborns, especially preterm and ill babies) against pain. In this trial it will be investigated if the prophylactic use of paracetamol will close the ductus arteriosus without observing any harmful side effects.
How does the study work?
The TREOCAPA study has two parts: Phase II and Phase III.
| Phase II | Phase III | |
|---|---|---|
| Study group | 30 preterm infants born at a gestational age of 23 to 26 weeks. | 396 preterm infants born at a gestational age of 23-26 weeks and 398 preterm infants of 27-28 weeks. |
| Aim | Define the minimum dose of paracetamol that is able to close the ductus arteriosus before or at day 7 after birth in preterm infants born at a gestational age of 23 to 28 weeks. | Find out whether the prophylactic use of paracetamol in preterm babies during their first five days of life is safe and effective in reducing risk of death or severe complications of prematurity, like brain bleedings, pulmonary lesions or eye problems, by preventing a persistent ductus arteriosus.
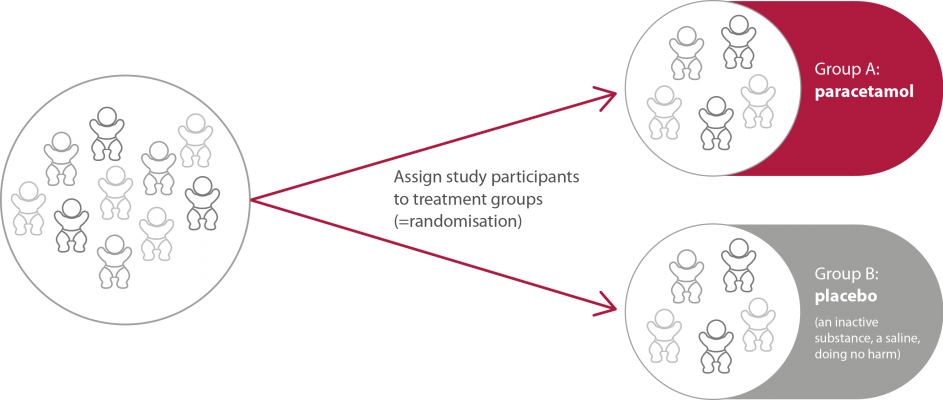
In this phase two different treatment groups will be compared to each other. Therefore, all patients will be allocated to one of two groups (figure 2). |
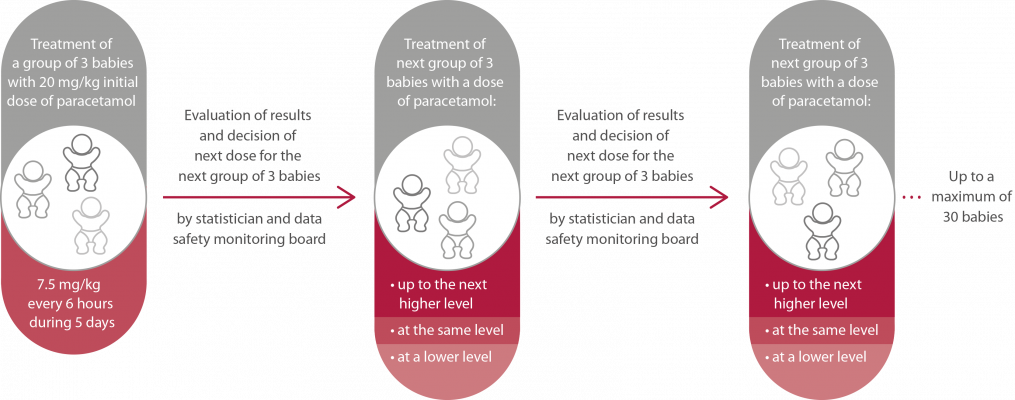
Figure 1. Study procedure in phase II of the study
Figure 2. Allocation of the participants in the study group in phase III
The role of EFCNI in TREOCAPA
EFCNI ensures public and patient involvement (PPI) during all phases of the TREOCAPA clinical trial and is responsible for the coordination and management of a Parental Advisory Board (PAB). We want to make sure that the parents of preterm babies have a say, are informed and reassured throughout the whole process since, in the end, the results will have a direct effect on their lives and those of their children. Moreover, EFCNI provides input on trial materials and is responsible for the communication strategy. The objectives of the communication strategy are:
- To engage all key stakeholders throughout the lifecycle of the study
- To increase the key stakeholders’ knowledge of PDA and how acetaminophen can have a positive preventive impact on this
- To increase support for this trial in the prophylactic treatment of acetaminophen and generally, for clinical trials in neonates
- To make the results of the study understandable and relevant to all key stakeholders
EFCNI works closely with the study team in France and other project partners to achieve these objectives through several different tasks. The partners we are working closely with are as follows:
TREOCAPA-LT
Even early interventions can have long-term effects on a person’s health outcomes, and thus it is essential, to expand the area of observation to a more long-term perspective. The TREOCAPA-LT study aims to investigate neurological development of the TREOCAPA participants at 24 months. Find more information by clicking on the button below:
Paediatric trials: industry and non-industry coming together
What are the differences between these two types of trials? What are the benefits of working together? Questions that may not be so easy to answer at first glance. This article gives an overview of both types of trial and the ways in which they work together, including the prominence of the patient voice.
Factsheet "Clinical Trials in Newborns"
In our new factsheet, we are offering an overview of important terms and definitions, as well as chances and challenges of clinical trials in newborns. Additionally, we explain the design of a clinical trial with the help of a practical example – the TREOCAPA study. Download your copy now!
Explanatory Video Series On Clinical Trials in Newborns
In this three-part explanatory video series, you will learn what a clinical trial is and which terms are important when it comes to clinical trials. You will also find information about the importance and objectives of conducting clinical trials in newborns. In addition, we will explain the design of a clinical trial with the help of the #TREOCAPA study.
Parent Video on experiences with the TREOCAPA study
How does it feel like for parents to take part in a clinical trial with their newborn baby? Thanks to four families who took part in the TREOCAPA study and have shared their experiences with us, we can now gain an insight into this challenging situation. In this video, you can learn more about:
- the experiences of the participating families with the TREOCAPA study
- if the parents would recommend other families and their babies to take part in a neonatal clinical trial, and if so, why?
- the scientific value of the TREOCAPA study according to the participating families
- next steps or follow-up actions that participating families would like to see once the TREOCAPA results are published and the trial is completed
We thank all the parents and families who shared their very valuable experiences with us in these videos! Together we can improve neonatal care and advance medicine in the field of neonatology.
Music:0081. Uplifting Piano – AShamaluevMusic
Parent Video Series - Social Media Clips
This four-part parent video series refers to the full-length video version published above. In 4 short clips, 2 answers from each of the participating parents/families to the following questions are summarised:
Clip 1: What were your experiences with the TREOCAPA study?
Clip 2: Would you recommend other parents and their babies to take part in a neonatal clinical trial, and if so, why?
Clip 3: In your opinion, what is the scientific value of the TREOCAPA study?
Clip 4: Once the TREOCAPA results are published and the trial is completed, are there any next steps or follow-up actions that you would like to see?
Music:0081. Uplifting Piano – AShamaluevMusic
Parent Quote Cards
Quote Card 1:
What do parents who have taken part in a clinical trial with their newborn baby wish for?
A family from Switzerland who took part in the TREOCAPA study shares their thoughts on this and emphasises the importance of:
- preparing and communicating the study results in a target group-orientated way for a lay audience
- providing parents with comprehensive follow-up information on the effects of the study results for the future care of preterm born babies
Quote Card 2:
What is important for parents when they decide to participate in a clinical trial with their newborn baby?
A family from France who took part in the TREOCAPA study shares their thoughts on this and emphasises the importance of:
- establishing a relationship of trust between parents and the study investigator
- fully explaining the individual study steps and giving parents the feeling that they are acting based on an informed decision
Thank You For Your Participation In The TREOCAPA Study
As the recruitment period of the TREOCAPA study is over, the entire study team would like to express our heartfelt thanks to all the participants and their parents for their participation and engagement in this research with a Thank You Letter.
It was only thanks to your contribution that we were able to perform this essential investigation. Your collected test results will now be analysed over the course of several months and lead to new findings and publications in this field.
Note about funding
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777389.
The Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.